Optune®
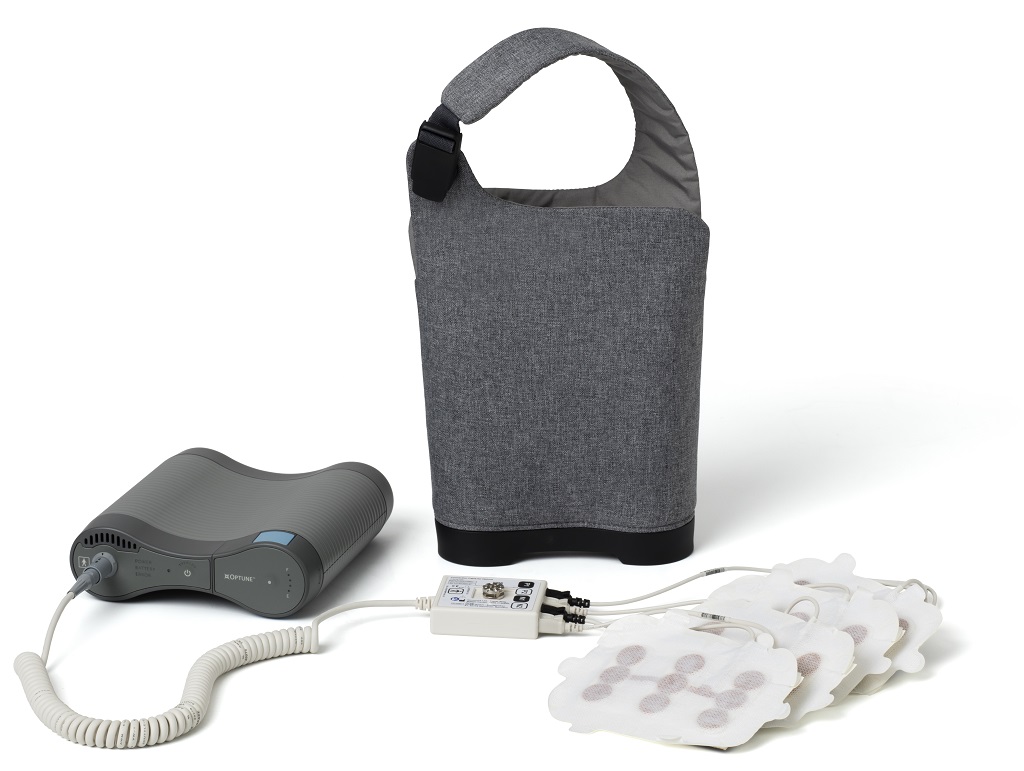
Messino Cancer Centers is now certified to prescribe Optune® for the treatment of newly diagnosed and recurrent glioblastoma (GBM). This wearable and portable medical device is the first FDA-approved treatment in more than a decade for newly diagnosed GBM.
How it works
Optune creates low-intensity alternating electric fields – called Tumor Treating Fields (TTFields) – which potentially slow or stop cell division leading to cell death. Because TTFields do not enter the bloodstream like a drug, TMZ (temozolomide chemotherapy)-related side effects for newly diagnosed patients can be minimized. In clinical trials the most common device related adverse events when using Optune alone were scalp irritation from device use and headache.
